Membrane-induced lever arm expansion allows myosin VI to walk with large and variable step sizes
2012.08.30Yu, C., Lou, J., Wu, J., Pan, L., Feng, W., & Zhang, M. (2012). Journal of Biological Chemistry, 287(42), 35021-35035.
Myosin VI, the only known minus-ended actin filament-dependent motor, plays diverse cellular roles both as a processive motor and as a mechanical anchor. Although myosin VI has a short lever arm containing only one “IQ-motif” and a unique insertion for CaM binding, the motor walks with large and variable step sizes of ∼30–36 nm. Here, we show that the previously predicted coiled-coil domain immediately following the IQ-motifs (referred to as the lever arm extension (LAE)) adopts a stable monomeric, three-helix bundle fold in solution. Importantly, the LAE can undergo reversible, lipid membrane-dependent conformational changes. Upon exposure to lipid membranes, the LAE adopts a partially extended rod shape, and the removal of lipids from the LAE converts it back into the compact helix bundle structure. Molecular dynamics simulations indicate that lipid membrane binding may initiate unfolding and thereby trigger the LAE expansion. This reversible, lipid membrane-dependent expansion of the LAE provides a mechanistic base for myosin VI to walk with large and variable step sizes.
- Recommend
-
2025-10-22
IQSEC2/BRAG1 may modulate postsynaptic density assembly through Ca2+-induced phase separation.
-
2025-08-22
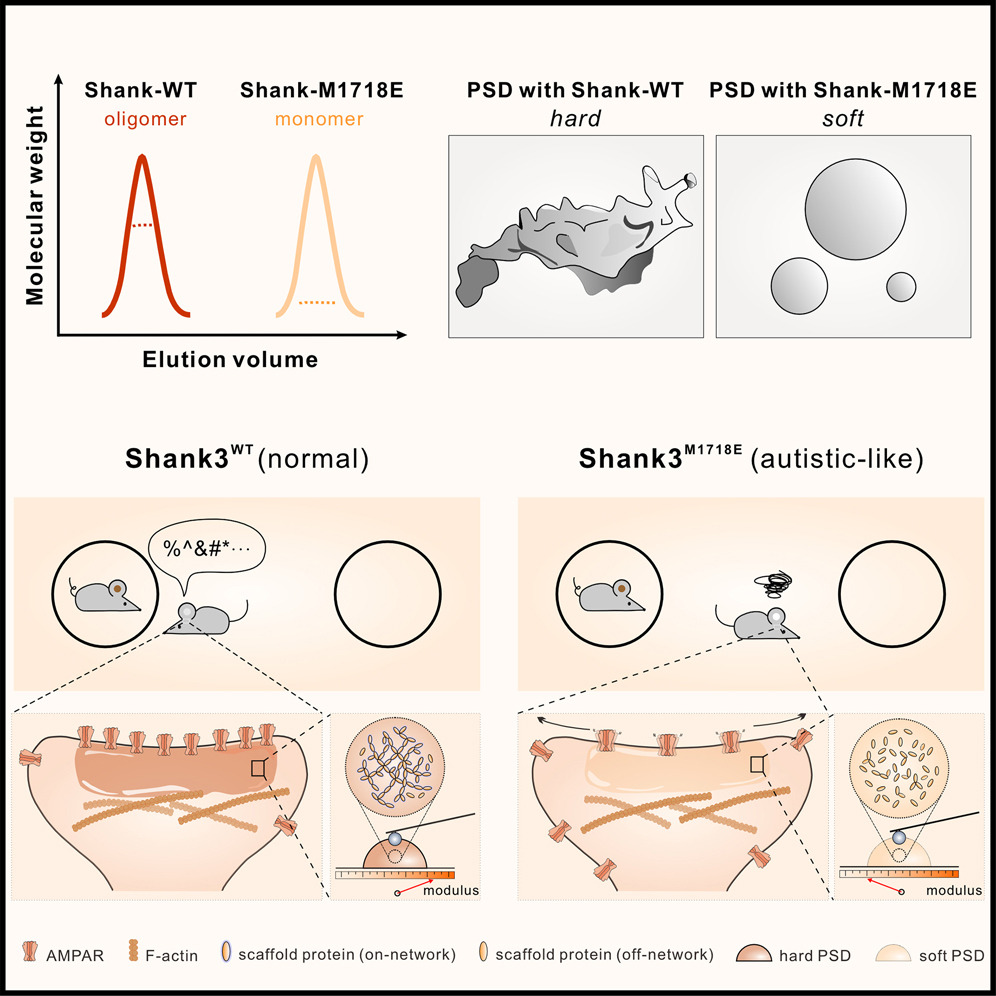
Shank3 oligomerization governs material properties of the postsynaptic density condensate and synaptic plasticity.
-
2025-08-21
Modulating synaptic glutamate receptors by targeting network nodes of the postsynaptic density condensate.
-
2025-08-19
Current practices in the study of biomolecular condensates: a community comment.
-
2025-06-10
Phase separation instead of binding strength determines target specificities of MAGUKs.

