Internally deleted human tRNA synthetase suggests evolutionary pressure for repurposing
2012.09.05Xu, Z., Wei, Z., Zhou, J. J., Ye, F., Lo, W. S., Wang, F., ... Zhang, M., & Schimmel, P. (2012). Structure, 20(9), 1470-1477.
Aminoacyl-tRNA synthetases (AARSs) catalyze aminoacylation of tRNAs in the cytoplasm. Surprisingly, AARSs also have critical extracellular and nuclear functions. Evolutionary pressure for new functions might be manifested by splice variants that skip only an internal catalytic domain (CD) and link noncatalytic N- and C-terminal polypeptides. Using disease-associated histidyl-tRNA synthetase (HisRS) as an example, we found an expressed 171-amino acid protein (HisRSΔCD) that deleted the entire CD, and joined an N-terminal WHEP to the C-terminal anticodon-binding domain (ABD). X-ray crystallography and three-dimensional NMR revealed the structures of human HisRS and HisRSΔCD. In contrast to homodimeric HisRS, HisRSΔCD is monomeric, where rupture of the ABD’s packing with CD resulted in a dumbbell-like structure of flexibly linked WHEP and ABD domains. In addition, the ABD of HisRSΔCD presents a distinct local conformation. This natural internally deleted HisRS suggests evolutionary pressure to reshape AARS tertiary and quaternary structures for repurposing.
- Recommend
-
2025-10-22
IQSEC2/BRAG1 may modulate postsynaptic density assembly through Ca2+-induced phase separation.
-
2025-08-22
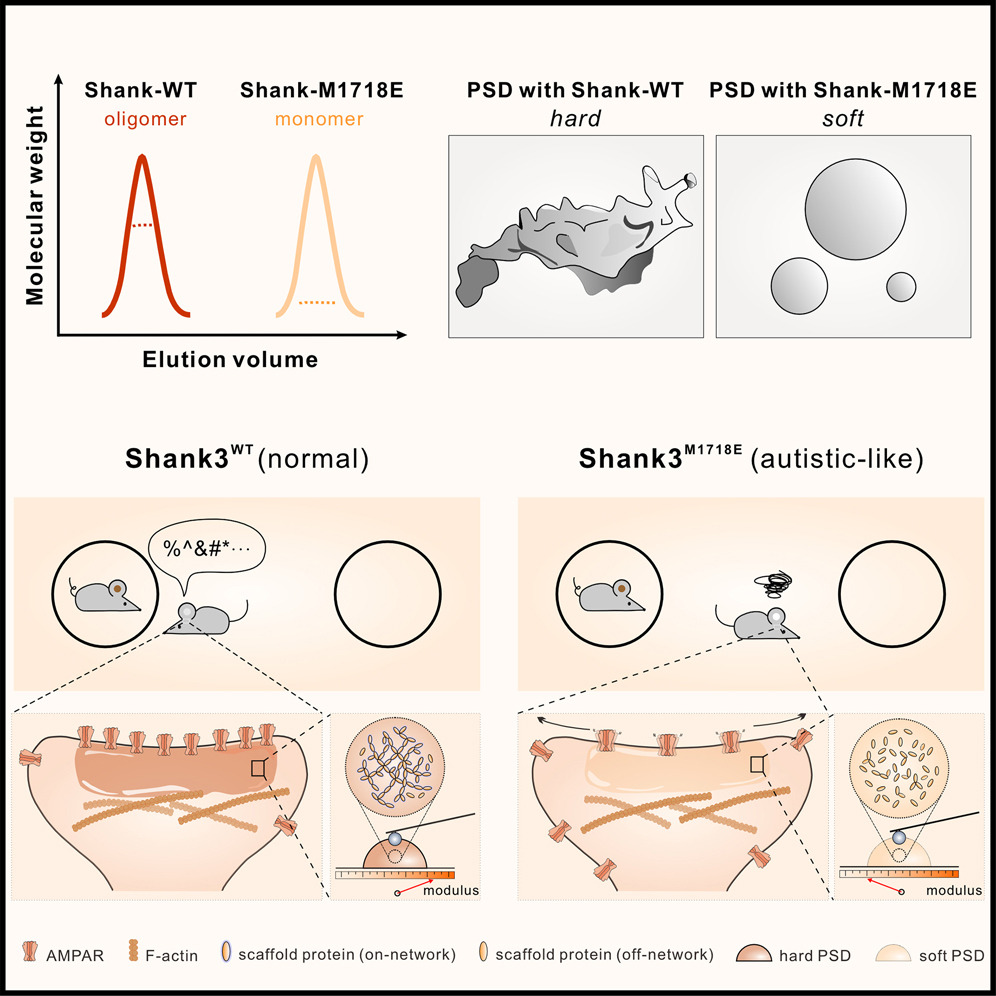
Shank3 oligomerization governs material properties of the postsynaptic density condensate and synaptic plasticity.
-
2025-08-21
Modulating synaptic glutamate receptors by targeting network nodes of the postsynaptic density condensate.
-
2025-08-19
Current practices in the study of biomolecular condensates: a community comment.
-
2025-06-10
Phase separation instead of binding strength determines target specificities of MAGUKs.

