Leucine-rich repeat kinase 2 binds to neuronal vesicles through protein interactions mediated by its C-terminal WD40 domain
2014.05.20Piccoli, G., Onofri, F., Cirnaru, M. D., Kaiser, C. J., Jagtap, P., Kastenmüller, A., Zhang, M., ... & Gloeckner, C. J. (2014). Molecular and cellular biology, 34(12), 2147-2161.
Mutations in the leucine-rich repeat kinase 2 gene (LRRK2) are associated with familial and sporadic Parkinson's disease (PD). LRRK2 is a complex protein that consists of multiple domains, including predicted C-terminal WD40 repeats. In this study, we analyzed functional and molecular features conferred by the WD40 domain. Electron microscopic analysis of the purified LRRK2 C-terminal domain revealed doughnut-shaped particles, providing experimental evidence for its WD40 fold. We demonstrate that LRRK2 WD40 binds and sequesters synaptic vesicles via interaction with vesicle-associated proteins. In fact, a domain-based pulldown approach combined with mass spectrometric analysis identified LRRK2 as being part of a highly specific protein network involved in synaptic vesicle trafficking. In addition, we found that a C-terminal sequence variant associated with an increased risk of developing PD, G2385R, correlates with a reduced binding affinity of LRRK2 WD40 to synaptic vesicles. Our data demonstrate a critical role of the WD40 domain within LRRK2 function.
- Recommend
-
2025-10-22
IQSEC2/BRAG1 may modulate postsynaptic density assembly through Ca2+-induced phase separation.
-
2025-08-22
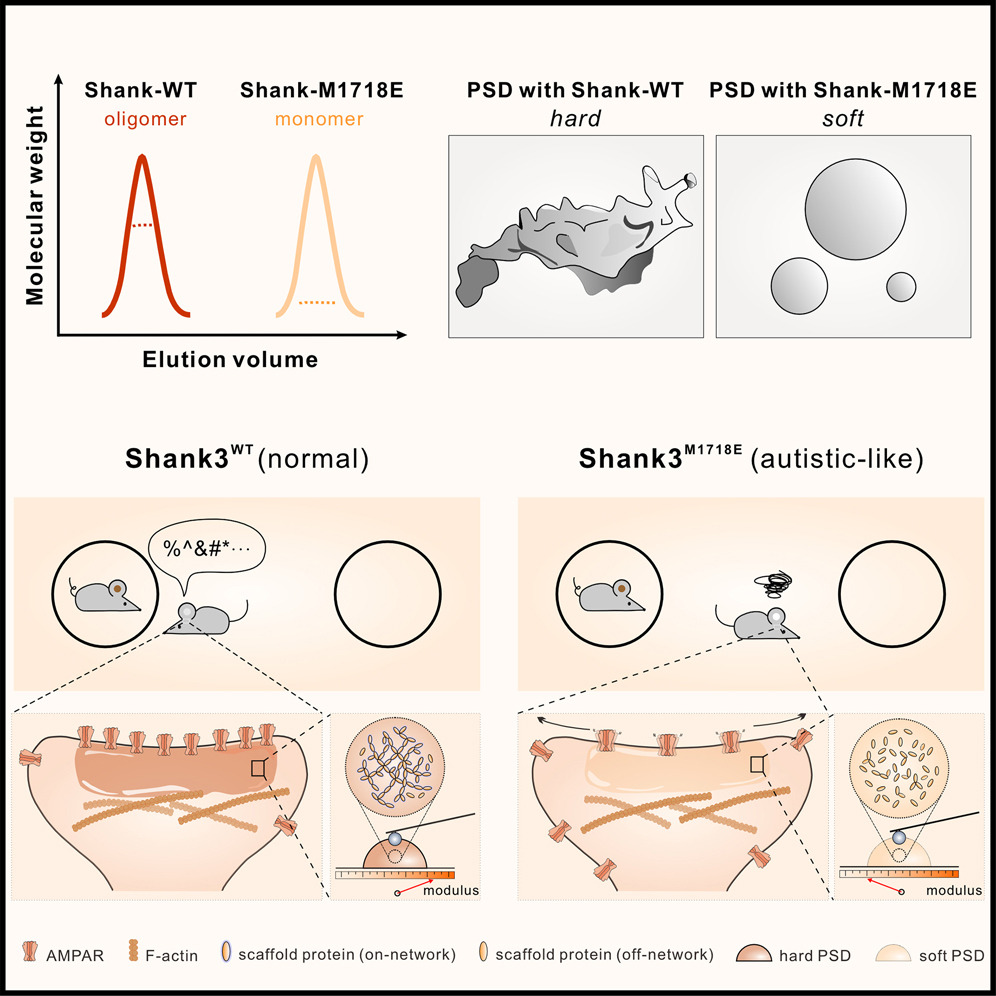
Shank3 oligomerization governs material properties of the postsynaptic density condensate and synaptic plasticity.
-
2025-08-21
Modulating synaptic glutamate receptors by targeting network nodes of the postsynaptic density condensate.
-
2025-08-19
Current practices in the study of biomolecular condensates: a community comment.
-
2025-06-10
Phase separation instead of binding strength determines target specificities of MAGUKs.

