Structural basis of the binding of Merlin FERM domain to the E3 ubiquitin ligase substrate adaptor DCAF1
2014.04.04Li, Y., Wei, Z., Zhang, J., Yang, Z., & Zhang, M. (2014). Journal of Biological Chemistry, 289(21), 14674-14681.
The tumor suppressor gene Nf2 product, Merlin, plays vital roles in controlling proper development of organ sizes by specifically binding to a large number of target proteins localized both in cytoplasm and nuclei. The FERM domain of Merlin is chiefly responsible for its binding to target proteins, although the molecular basis governing these interactions are poorly understood due to lack of structural information. Here, we report the crystal structure of the Merlin FERM domain in complex with its binding domain derived from the E3 ubiquitin ligase substrate adaptor DCAF1 (also known as VPRBP). Unlike target binding modes found in ERM proteins, the Merlin-FERM binding domain of DCAF1 folds as a β-hairpin and binds to the α1/β5-groove of the F3 lobe of Merlin-FERM via extensive hydrophobic interactions. In addition to providing the first structural glimpse of a Merlin-FERM·target complex, the structure of the Merlin·DCAF1 complex is likely to be valuable for understanding the interactions of Merlin with its binding partners other than DCAF1.
- Recommend
-
2025-10-22
IQSEC2/BRAG1 may modulate postsynaptic density assembly through Ca2+-induced phase separation.
-
2025-08-22
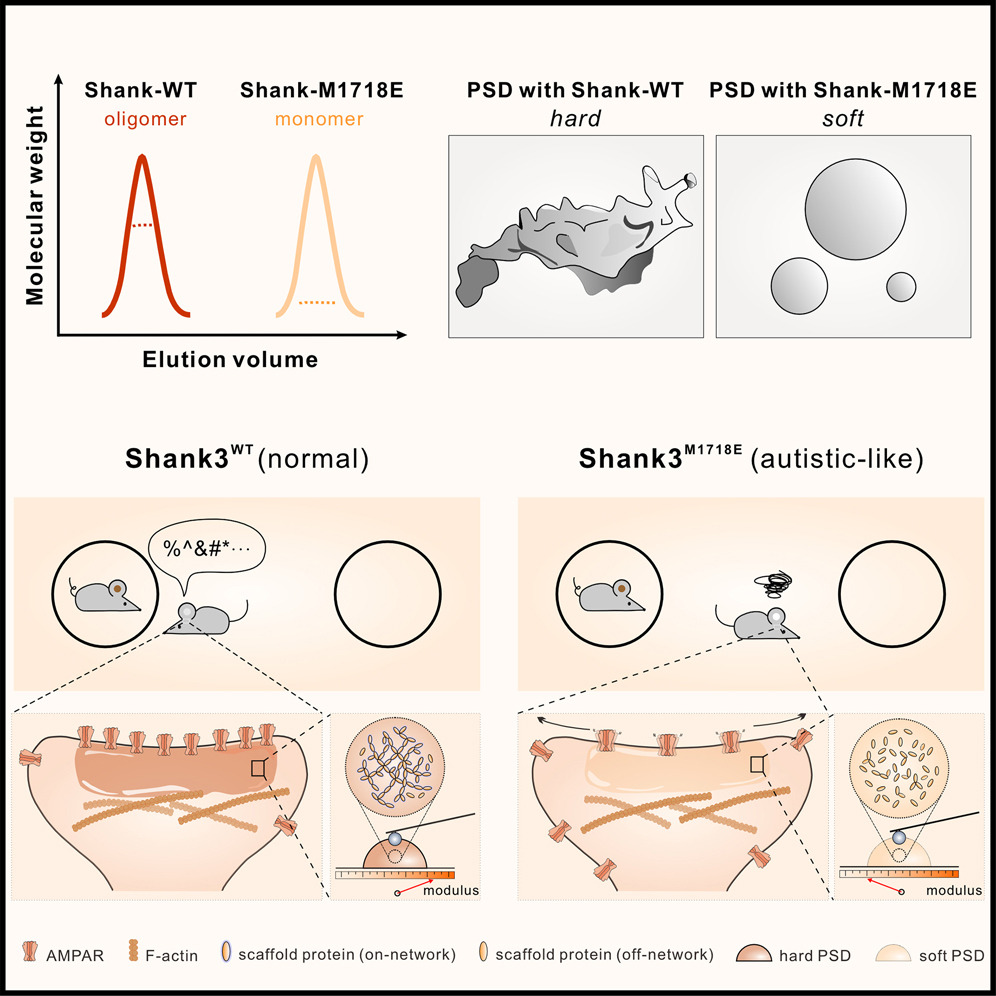
Shank3 oligomerization governs material properties of the postsynaptic density condensate and synaptic plasticity.
-
2025-08-21
Modulating synaptic glutamate receptors by targeting network nodes of the postsynaptic density condensate.
-
2025-08-19
Current practices in the study of biomolecular condensates: a community comment.
-
2025-06-10
Phase separation instead of binding strength determines target specificities of MAGUKs.

