Alternative splicing creates two new architectures for human tyrosyl-tRNA synthetase
2016.01.14Wei, Z., Xu, Z., Liu, X., Lo, W. S., Ye, F., Lau, C. F., ... Zhang, M., & Schimmel, P. (2016). Nucleic Acids Research, 44(3), 1247-1255.
Many human tRNA synthetases evolved alternative functions outside of protein synthesis. These functions are associated with over 200 splice variants (SVs), most of which are catalytic nulls that engender new biology. While known to regulate non-translational activities, little is known about structures resulting from natural internal ablations of any protein. Here, we report analysis of two closely related, internally deleted, SVs of homodimeric human tyrosyl-tRNA synthetase (TyrRS). In spite of both variants ablating a portion of the catalytic core and dimer-interface contacts of native TyrRS, each folded into a distinct stable structure. Biochemical and nuclear magnetic resonance (NMR) analysis showed that the internal deletion of TyrRSΔE2–4 SV gave an alternative, neomorphic dimer interface ‘orthogonal’ to that of native TyrRS. In contrast, the internal C-terminal splice site of TyrRSΔE2–3 prevented either dimerization interface from forming, and yielded a predominantly monomeric protein. Unlike ubiquitous TyrRS, the neomorphs showed clear tissue preferences, which were distinct from each other. The results demonstrate a sophisticated structural plasticity of a human tRNA synthetase for architectural reorganizations that are preferentially elicited in specific tissues.
- Recommend
-
2025-10-22
IQSEC2/BRAG1 may modulate postsynaptic density assembly through Ca2+-induced phase separation.
-
2025-08-22
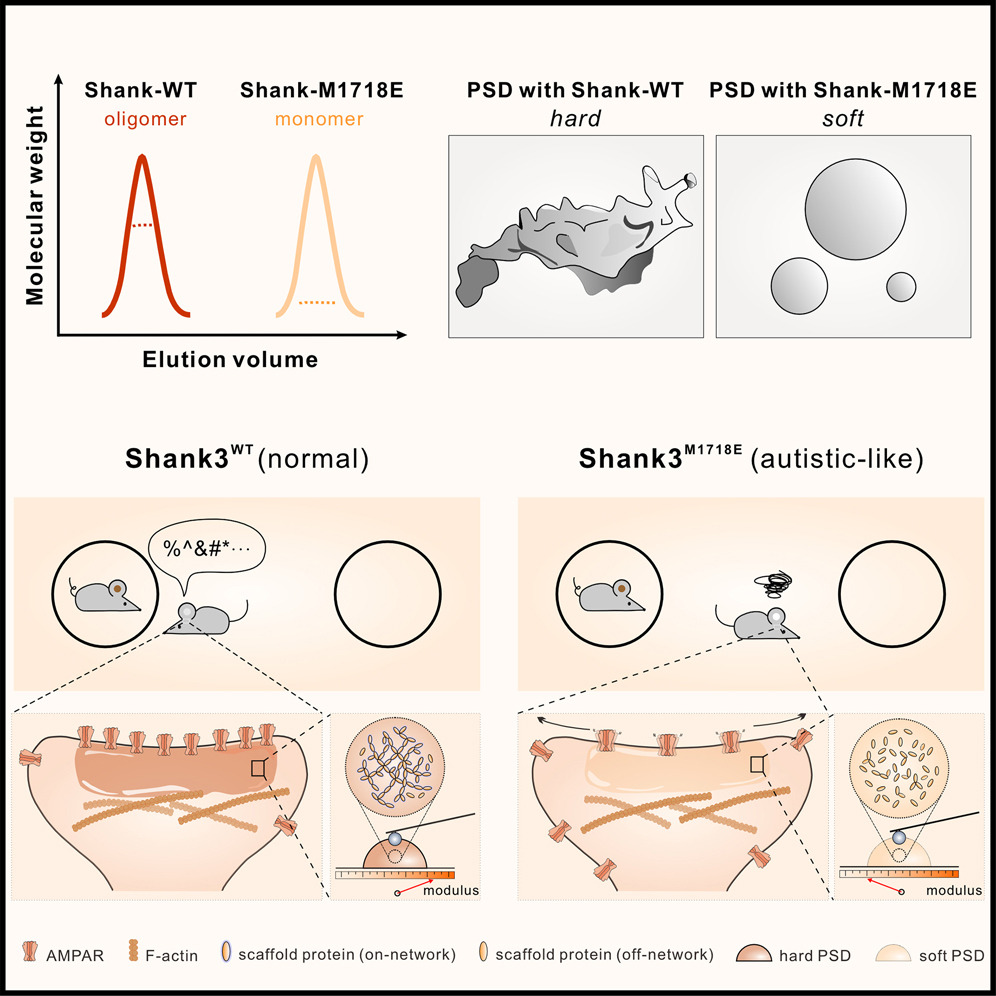
Shank3 oligomerization governs material properties of the postsynaptic density condensate and synaptic plasticity.
-
2025-08-21
Modulating synaptic glutamate receptors by targeting network nodes of the postsynaptic density condensate.
-
2025-08-19
Current practices in the study of biomolecular condensates: a community comment.
-
2025-06-10
Phase separation instead of binding strength determines target specificities of MAGUKs.

