Structure of the MORN4/Myo3a tail complex reveals MORN repeats as protein binding modules
2019.09.03Li, J., Liu, H., Raval, M. H., Wan, J., Yengo, C. M., Liu, W., & Zhang, M. (2019). Structure, 27(9), 1366-1374.
Tandem repeats are basic building blocks for constructing proteins with diverse structures and functions. Compared with extensively studied α-helix-based tandem repeats such as ankyrin, tetratricopeptide, armadillo, and HEAT repeat proteins, relatively little is known about tandem repeat proteins formed by β hairpins. In this study, we discovered that the MORN repeats from MORN4 function as a protein binding module specifically recognizing a tail cargo binding region from Myo3a. The structure of the MORN4/Myo3a complex shows that MORN4 forms an extended single-layered β-sheet structure and uses a U-shaped groove to bind to the Myo3a tail with high affinity and specificity. Sequence and structural analyses further elucidated the unique sequence features for folding and target binding of MORN repeats. Our work establishes that the β-hairpin-based MORN repeats are protein-protein interaction modules.
- Recommend
-
2025-10-22
IQSEC2/BRAG1 may modulate postsynaptic density assembly through Ca2+-induced phase separation.
-
2025-08-22
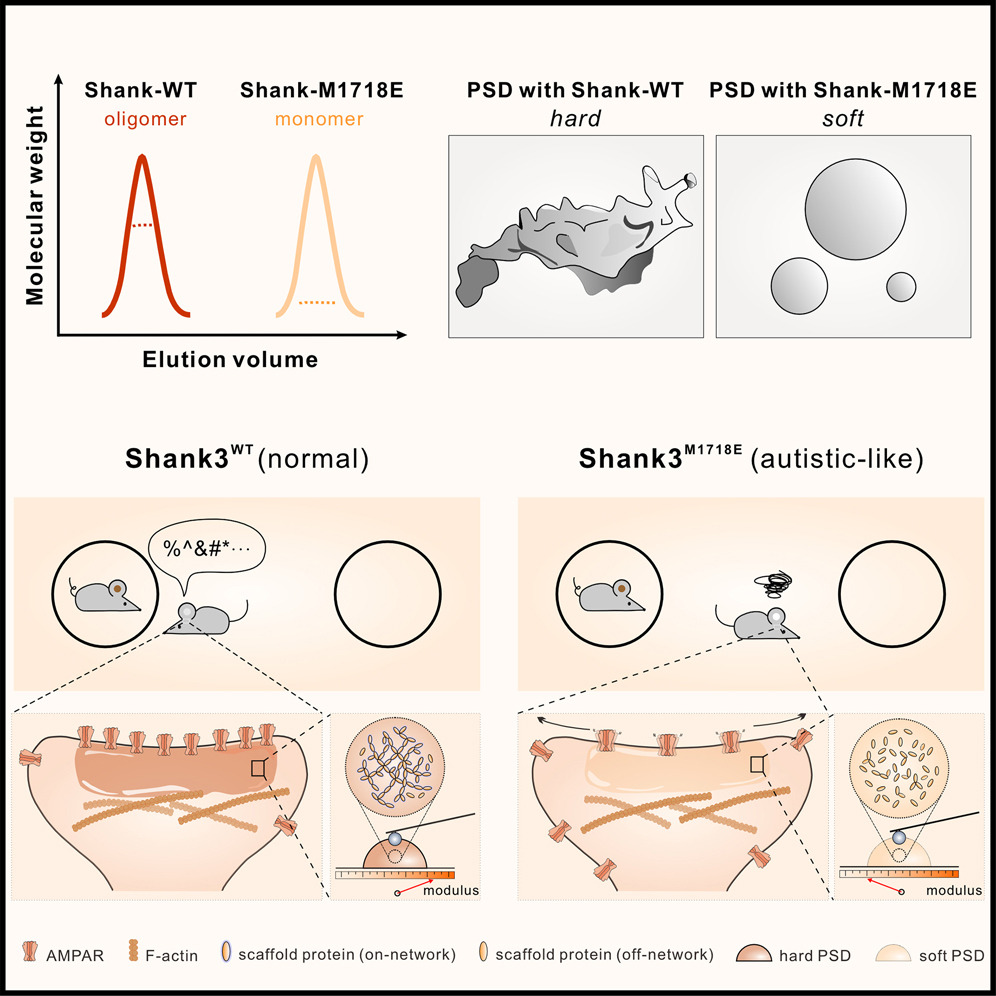
Shank3 oligomerization governs material properties of the postsynaptic density condensate and synaptic plasticity.
-
2025-08-21
Modulating synaptic glutamate receptors by targeting network nodes of the postsynaptic density condensate.
-
2025-08-19
Current practices in the study of biomolecular condensates: a community comment.
-
2025-06-10
Phase separation instead of binding strength determines target specificities of MAGUKs.

