Phase separation-mediated TARP/MAGUK complex condensation and AMPA receptor synaptic transmission
2019.11.06Zeng, M., Díaz-Alonso, J., Ye, F., Chen, X., Xu, J., Ji, Z., ... & Zhang, M. (2019). Neuron, 104(3), 529-543.
Transmembrane AMPA receptor (AMPAR) regulatory proteins (TARPs) modulate AMPAR synaptic trafficking and transmission via disc-large (DLG) subfamily of membrane-associated guanylate kinases (MAGUKs). Despite extensive studies, the molecular mechanism governing specific TARP/MAGUK interaction remains elusive. Using stargazin and PSD-95 as the representatives, we discover that the entire tail of stargazin (Stg_CT) is required for binding to PSD-95. The PDZ binding motif (PBM) and an Arg-rich motif upstream of PBM conserved in TARPs bind to multiple sites on PSD-95, thus resulting in a highly specific and multivalent stargazin/PSD-95 complex. Stargazin in complex with PSD-95 or PSD-95-assembled postsynaptic complexes form highly concentrated and dynamic condensates via phase separation, reminiscent of stargazin/PSD-95-mediated AMPAR synaptic clustering and trapping. Importantly, charge neutralization mutations in TARP_CT Arg-rich motif weakened TARP’s condensation with PSD-95 and impaired TARP-mediated AMPAR synaptic transmission in mice hippocampal neurons. The TARP_CT/PSD-95 interaction mode may have implications for understanding clustering of other synaptic transmembrane proteins.
- Recommend
-
2025-10-22
IQSEC2/BRAG1 may modulate postsynaptic density assembly through Ca2+-induced phase separation.
-
2025-08-22
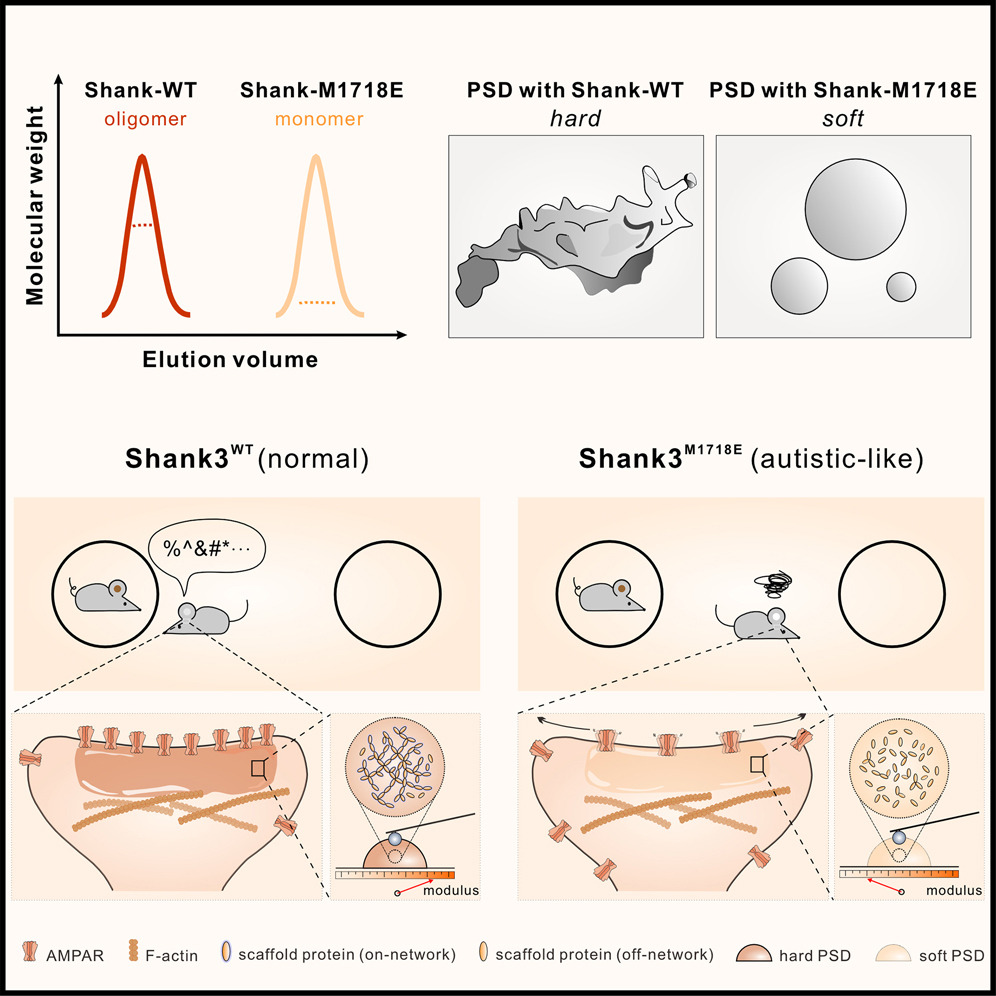
Shank3 oligomerization governs material properties of the postsynaptic density condensate and synaptic plasticity.
-
2025-08-21
Modulating synaptic glutamate receptors by targeting network nodes of the postsynaptic density condensate.
-
2025-08-19
Current practices in the study of biomolecular condensates: a community comment.
-
2025-06-10
Phase separation instead of binding strength determines target specificities of MAGUKs.

