Zhu, J., Shang, Y., & Zhang, M. (2016). Nature Reviews Neuroscience, 17(4), 209-223.
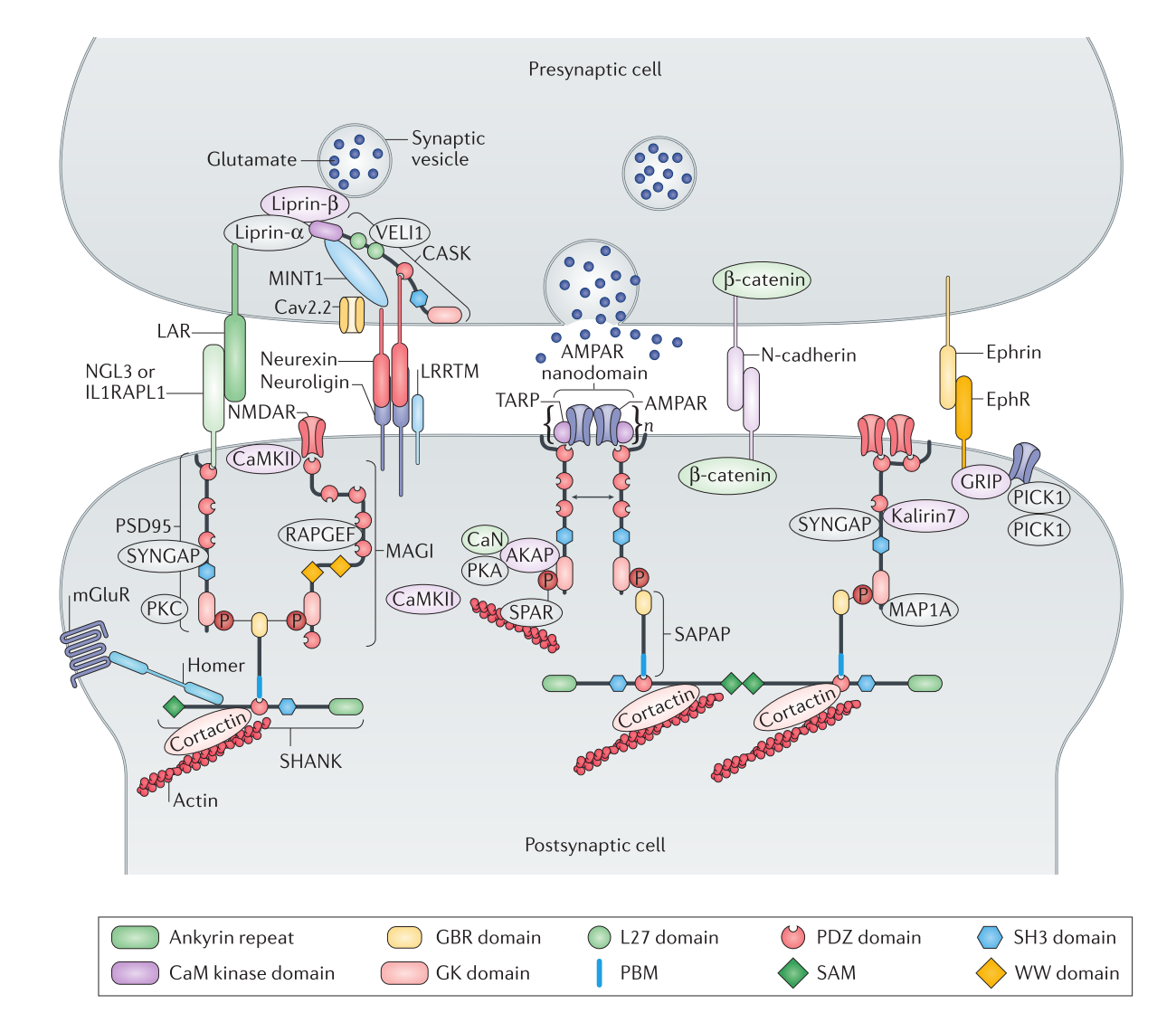
Membrane-associated guanylate kinases (MAGUKs) are a family of scaffold proteins that are highly enriched in synapses and are responsible for organizing the numerous protein complexes required for synaptic development and plasticity. Mutations in genes encoding MAGUKs and their interacting proteins can cause a broad spectrum of human psychiatric disorders. Here, we review MAGUK-mediated synaptic protein complex formation and regulation by focusing on findings from recent biochemical and structural investigations. These mechanistic-based studies show that the formation of MAGUK-organized complexes is often directly regulated by protein phosphorylation, suggesting a close connection between neuronal activity and the assembly of dynamic protein complexes in synapses.
Ji, Z., Li, H., Yang, Z., Huang, X., Ke, X., Ma, S., ... & Zhang, M. (2019). Cell reports, 26(8), 2064-2077.
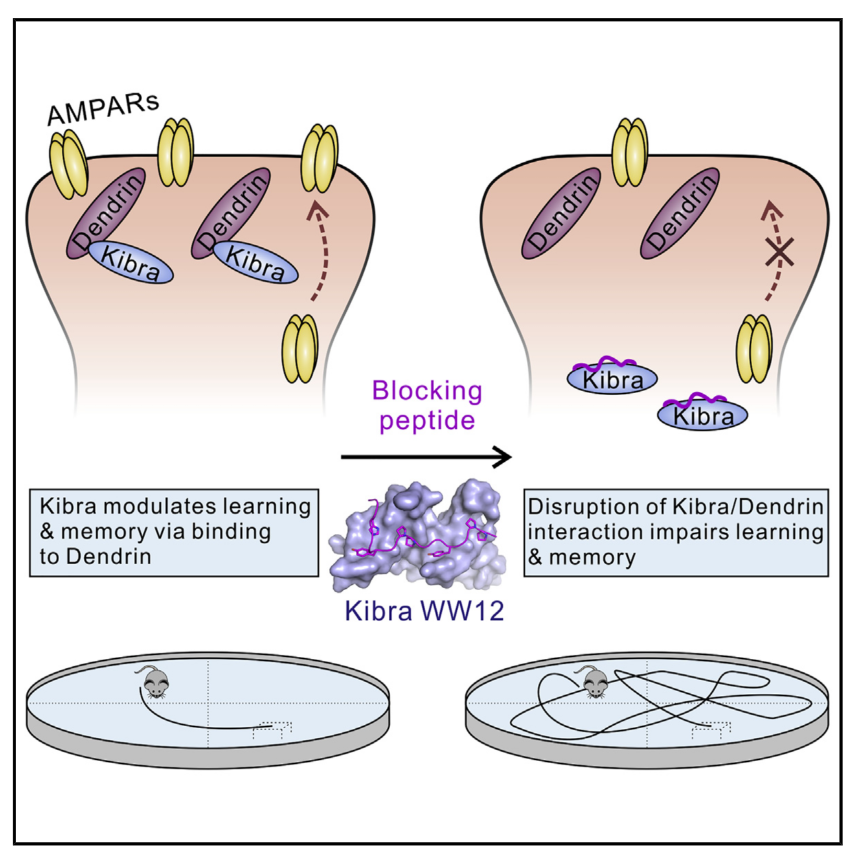
Kibra is a synaptic scaffold protein regulating learning and memory. Alterations of Kibra-encoding gene WWC1 cause various neuronal disorders, including Alzheimer’s disease and Tourette syndrome. However, the molecular mechanism underlying Kibra’s function in neurons is poorly understood. Here we discover that Kibra, via its N-terminal WW12 tandem domains, binds to a postsynaptic density enriched protein, Dendrin, with a nanomolar dissociation constant. On the basis of the structure of Kibra WW12 in complex with Dendrin PY motifs, we developed a potent peptide inhibitor capable of specifically blocking the binding between Kibra and Dendrin in neurons. Systematic administration of the inhibitory peptide attenuated excitatory synaptic transmission, completely blocked long-term potentiation induction, and impaired spatial learning and memory. A Kibra mutation found in Tourette syndrome patients causes defects in binding to Dendrin. Thus, Kibra can modulate spatial learning and memory via binding to Dendrin.
Zeng, M., Ye, F., Xu, J., and Zhang, M.(2018). Journal of Molecular Biology, 430(1), 69-86.
Discs large (DLG) MAGUKs are abundantly expressed in glutamatergic synapses, crucial for synaptic transmission, and plasticity by anchoring various postsynaptic components including glutamate receptors, downstream scaffold proteins and signaling enzymes. Different DLG members have shared structures and functions, but also contain unique features. How DLG family proteins function individually and cooperatively is largely unknown. Here, we report that PSD-95 PDZ3 directly couples with SH3–GK tandem in a PDZ ligand binding-dependent manner, and the coupling can promote PSD-95 dimerization and multimerization. Aided by sortase-mediated protein ligation and selectively labeling, we elucidated the PDZ3/SH3–GK conformational coupling mechanism using NMR spectroscopy. We further demonstrated that PSD-93, but not SAP102, can also undergo PDZ3 ligand binding-induced conformational coupling with SH3–GK and form homo-oligomers. Interestingly, PSD-95 and PSD-93 can also form ligand binding-induced hetero-oligomers, suggesting a cooperative assembly mechanism for the mega-N-methyl-d-aspartate receptor synaptic signaling complex. Finally, we provide evidence showing that ligand binding-induced conformational coupling between PDZ and SH3–GK is a common feature for other MAGUKs including CASK and PALS1.
Zhu, J., Zhou, Q., Shang, Y., Li, H., Peng, M., Ke, X., ... & Zhang, M. (2017). Cell reports, 21(13), 3781-3793.
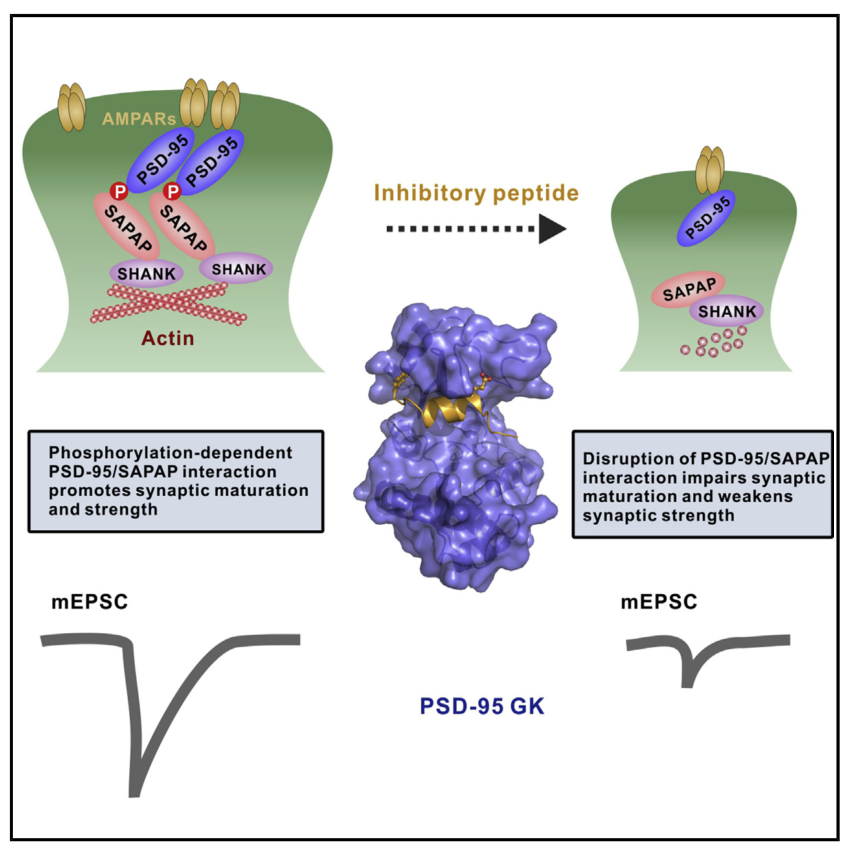
The PSD-95/SAPAP/Shank complex functions as the major scaffold in orchestrating the formation and plasticity of the post-synaptic densities (PSDs). We previously demonstrated that the exquisitely specific SAPAP/Shank interaction is critical for Shank synaptic targeting and Shank-mediated synaptogenesis. Here, we show that the PSD-95/SAPAP interaction, SAPAP synaptic targeting, and SAPAP-mediated synaptogenesis require phosphorylation of the N-terminal repeat sequences of SAPAPs. The atomic structure of the PSD-95 guanylate kinase (GK) in complex with a phosphor-SAPAP repeat peptide, together with biochemical studies, reveals the molecular mechanism underlying the phosphorylation-dependent PSD-95/SAPAP interaction, and it also provides an explanation of a PSD-95 mutation found in patients with intellectual disabilities. Guided by the structural data, we developed potent non-phosphorylated GK inhibitory peptides capable of blocking the PSD-95/SAPAP interaction and interfering with PSD-95/SAPAP-mediated synaptic maturation and strength. These peptides are genetically encodable for investigating the functions of the PSD-95/SAPAP interaction in vivo.
Ye, F., Kang, E., Yu, C., Qian, X., Jacob, F., Yu, C., ... & Zhang, M. (2017). Neuron, 96(5), 1041-1054.
Mutations of DISC1 (disrupted-in-schizophrenia 1) have been associated with major psychiatric disorders. Despite the hundreds of DISC1-binding proteins reported, almost nothing is known about how DISC1 interacts with other proteins structurally to impact human brain development. Here we solved the high-resolution structure of DISC1 C-terminal tail in complex with its binding domain of Ndel1. Mechanistically, DISC1 regulates Ndel1’s kinetochore attachment, but not its centrosome localization, during mitosis. Functionally, disrupting DISC1/Ndel1 complex formation prolongs mitotic length and interferes with cell-cycle progression in human cells, and it causes cell-cycle deficits of radial glial cells in the embryonic mouse cortex and human forebrain organoids. We also observed similar deficits in organoids derived from schizophrenia patient induced pluripotent stem cells (iPSCs) with a DISC1 mutation that disrupts its interaction with Ndel1. Our study uncovers a new mechanism of action for DISC1 based on its structure, and it has implications for how genetic insults may contribute to psychiatric disorders.