The structure of the PDZ3-SH3-GuK tandem of ZO-1 protein suggests a supramodular organization of the membrane-associated guanylate kinase (MAGUK) family scaffold protein core
2011.09.30Pan, L., Chen, J., Yu, J., Yu, H., & Zhang, M. (2011). Journal of Biological Chemistry, 286(46), 40069-40074.
Membrane-associated guanylate kinases (MAGUKs) are a large family of scaffold proteins that play essential roles in tethering membrane receptors, adhesion molecules, and macromolecular signaling complexes for tissue developments, cell-cell communications, and intracellular signal transductions. The defining feature of the MAGUK family scaffolds is that each member contains a conserved core consisting of a PSD-95/Dlg/ZO-1 (PDZ) domain, an Src homology 3 (SH3) domain, and a catalytically inactive guanylate kinase (GuK) domain arranged in tandem, although the structural features and functional implications of the PDZ-SH3-GuK tandem arrangement are unclear. The structure of the ZO-1 PDZ3-SH3-GuK tandem solved in this study reveals that the PDZ domain directly interacts with the SH3-GuK module, forming a structural supramodule with distinct target binding properties with respect to the isolated domains. Structure-based sequence analysis suggests that the PDZ-SH3-GuK tandems of other members of the MAGUK family also form supramodules.
- Recommend
-
2025-10-22
IQSEC2/BRAG1 may modulate postsynaptic density assembly through Ca2+-induced phase separation.
-
2025-08-22
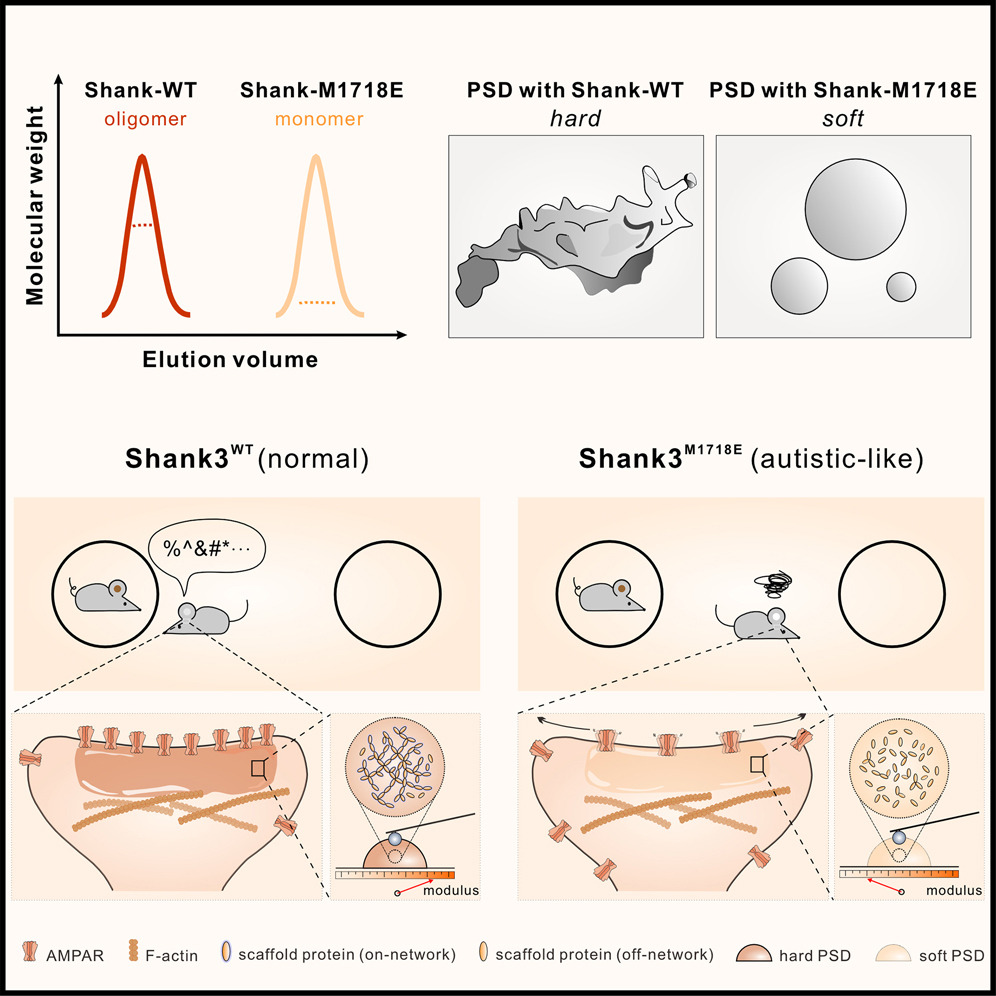
Shank3 oligomerization governs material properties of the postsynaptic density condensate and synaptic plasticity.
-
2025-08-21
Modulating synaptic glutamate receptors by targeting network nodes of the postsynaptic density condensate.
-
2025-08-19
Current practices in the study of biomolecular condensates: a community comment.
-
2025-06-10
Phase separation instead of binding strength determines target specificities of MAGUKs.

