An exquisitely specific PDZ/target recognition revealed by the structure of INAD PDZ3 in complex with TRP channel tail
2016.03.01Ye, F., Liu, W., Shang, Y., & Zhang, M. (2016). Structure, 24(3), 383-391.
The vast majority of PDZ domains are known to bind to a few C-terminal tail residues of target proteins with modest binding affinities and specificities. Such promiscuous PDZ/target interactions are not compatible with highly specific physiological functions of PDZ domain proteins and their targets. Here, we report an unexpected PDZ/target binding occurring between the scaffold protein inactivation no afterpotential D (INAD) and transient receptor potential (TRP) channel in Drosophila photoreceptors. The C-terminal 15 residues of TRP are required for the specific interaction with INAD PDZ3. The INAD PDZ3/TRP peptide complex structure reveals that only the extreme C-terminal Leu of TRP binds to the canonical αB/βB groove of INAD PDZ3. The rest of the TRP peptide, by forming a β hairpin structure, binds to a surface away from the αB/βB groove of PDZ3 and contributes to the majority of the binding energy. Thus, the INAD PDZ3/TRP channel interaction is exquisitely specific and represents a new mode of PDZ/target recognitions.
- Recommend
-
2025-10-22
IQSEC2/BRAG1 may modulate postsynaptic density assembly through Ca2+-induced phase separation.
-
2025-08-22
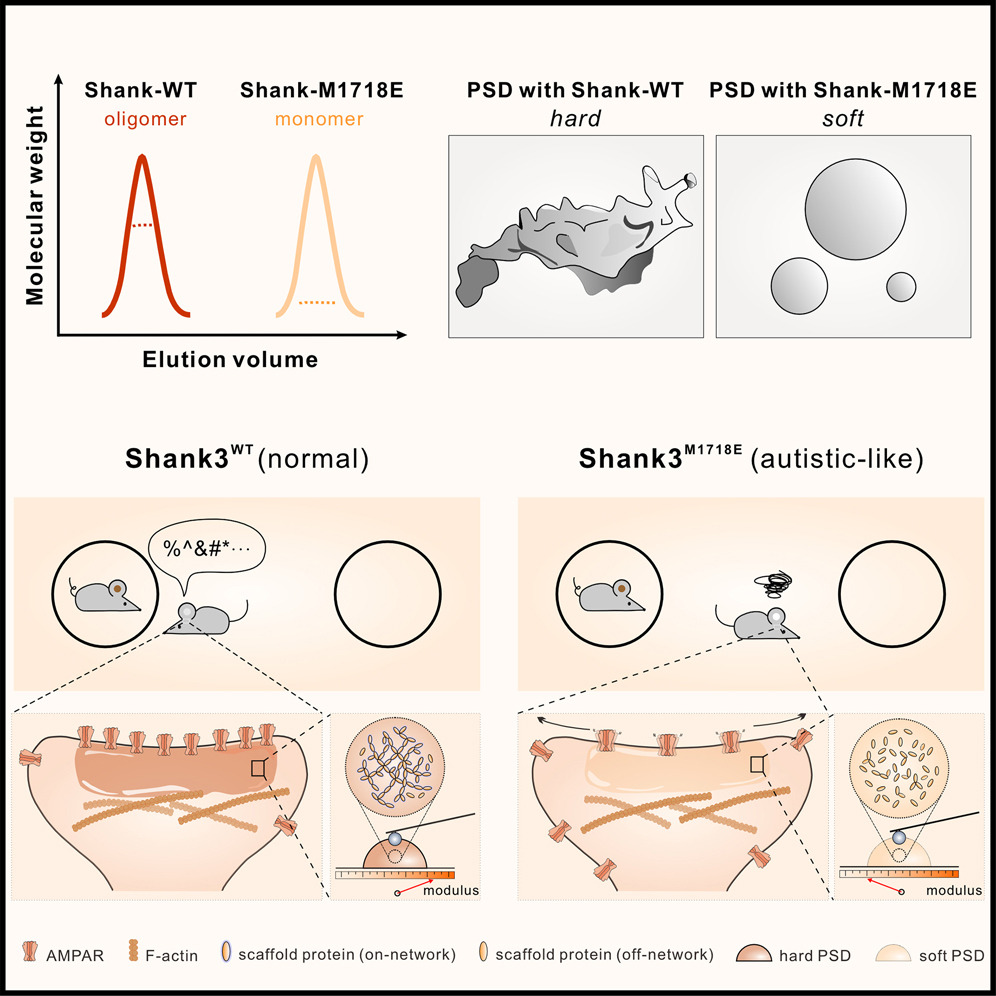
Shank3 oligomerization governs material properties of the postsynaptic density condensate and synaptic plasticity.
-
2025-08-21
Modulating synaptic glutamate receptors by targeting network nodes of the postsynaptic density condensate.
-
2025-08-19
Current practices in the study of biomolecular condensates: a community comment.
-
2025-06-10
Phase separation instead of binding strength determines target specificities of MAGUKs.

