Anchoring high concentrations of SynGAP at postsynaptic densities via liquid-liquid phase separation
2017.06.23Zeng, M., Bai, G., & Zhang, M. (2019). Small GTPases, 10(4), 296-304.
SynGAP, encoded by SYNGAP1, is a Ras/Rap GTPase activator specifically expressed in the nervous systems. SynGAP is one of the most abundant proteins in the postsynaptic densities (PSDs) of excitatory synapses and acts as a critical synaptic activity brake by tuning down synaptic GTPase activities. Mutations of SYNGAP1 have been frequently linked to brain disorders including intellectual disability, autisms, and seizure. SynGAP has been shown to undergo fast dispersions from synapses in response to stimulations, a strategy that neurons use to control the specific activities of the enzyme within the tiny, semi-open compartments in dendritic spines. However, the mechanism governing the activity-dependent synaptic localization modulations of SynGAP is poorly understood. It has been shown recently that SynGAP α1, via specifically binding to PSD-95, can undergo liquid-liquid phase separation forming membraneless, condensed protein-rich sub-compartments. This phase transition-mediated, PSD-95-dependent synaptic enrichment of SynGAP α1 not only suggests a dynamic anchoring mechanism of the protein within the PSD, but also implies a new model for the PSD formation in living neurons.
- Recommend
-
2025-10-22
IQSEC2/BRAG1 may modulate postsynaptic density assembly through Ca2+-induced phase separation.
-
2025-08-22
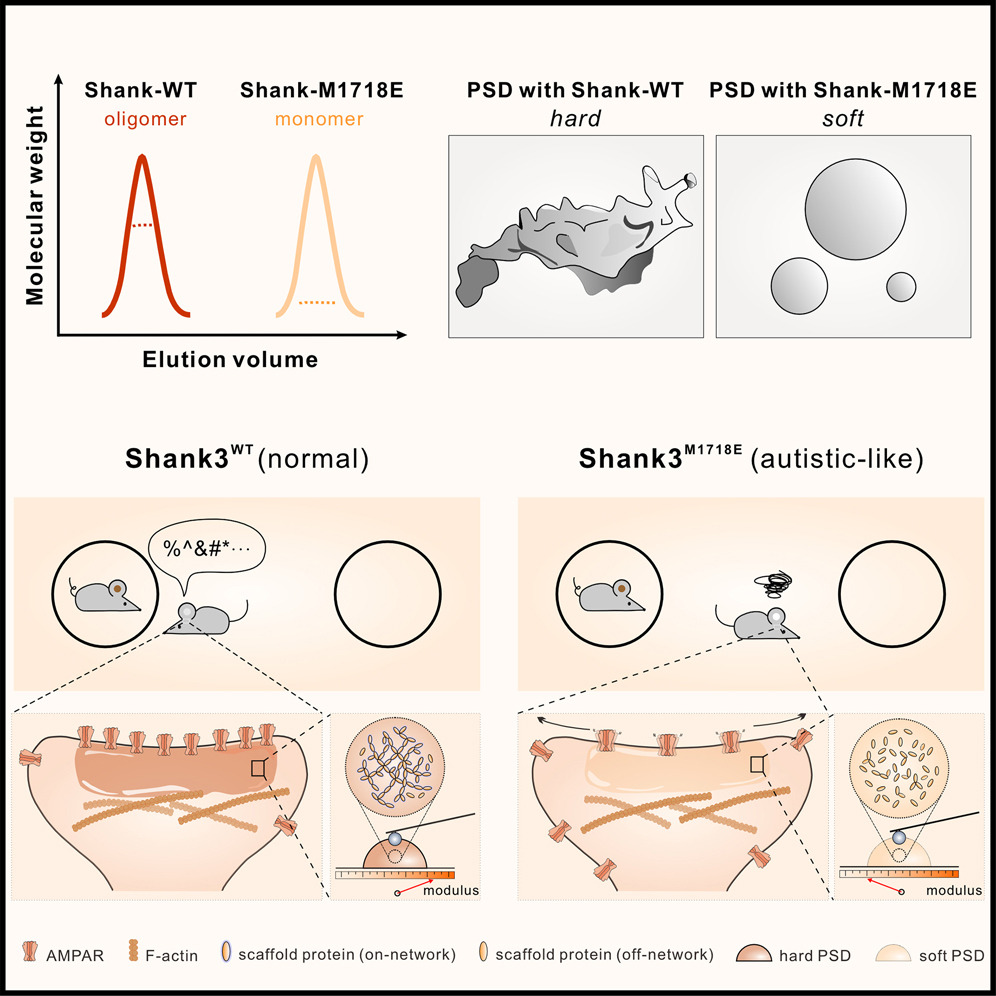
Shank3 oligomerization governs material properties of the postsynaptic density condensate and synaptic plasticity.
-
2025-08-21
Modulating synaptic glutamate receptors by targeting network nodes of the postsynaptic density condensate.
-
2025-08-19
Current practices in the study of biomolecular condensates: a community comment.
-
2025-06-10
Phase separation instead of binding strength determines target specificities of MAGUKs.

